A lot of new features and new sections in Biotechgate will be launched with a new software release end of October 2019. In this article, Jost Renggli, COO of Venture Valuation / Biotechgate and responsible for the development of the new version, provides a preview and details about the expected new features of the software.
The launch of a new version of Biotechgate with a lot of new features has been announced. What makes this version different from previous releases and what can be expected?
Jost Renggli: The upcoming new version of Biotechgate is the biggest release in more than six years. Main changes include the introduction of a new diagnostics section, a newly designed area for the search of assets and licensing opportunities as well as the ability to see all search results on a map. Furthermore, all search forms have been completely re-developed and a lot of details have been improved and enhanced.
How will the new diagnostic and asset search look like?
We will introduce a new section that enables searching for specific assets. This will make it easier to differentiate between therapeutics, medical devices, and technologies. In addition, a dedicated area will allow for searching for diagnostics. It will also be possible to filter the more than 55,000 companies in Biotechgate by the type of assets if they provide, e.g. to identify Biotech manufacturer offering diagnostics.
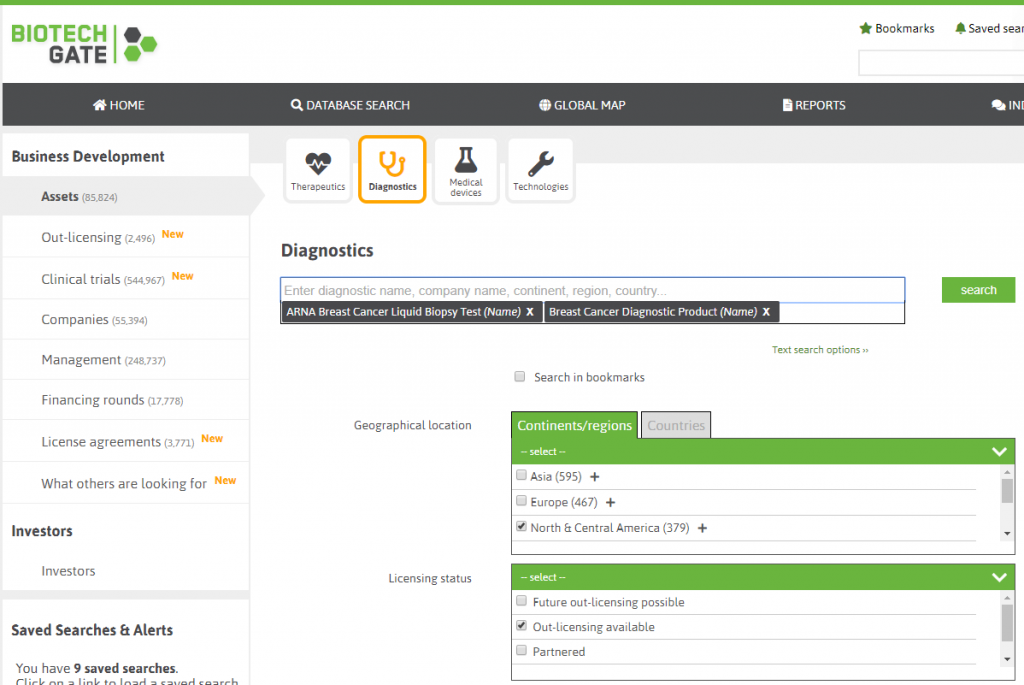
What about the new “map search”, how will this work?
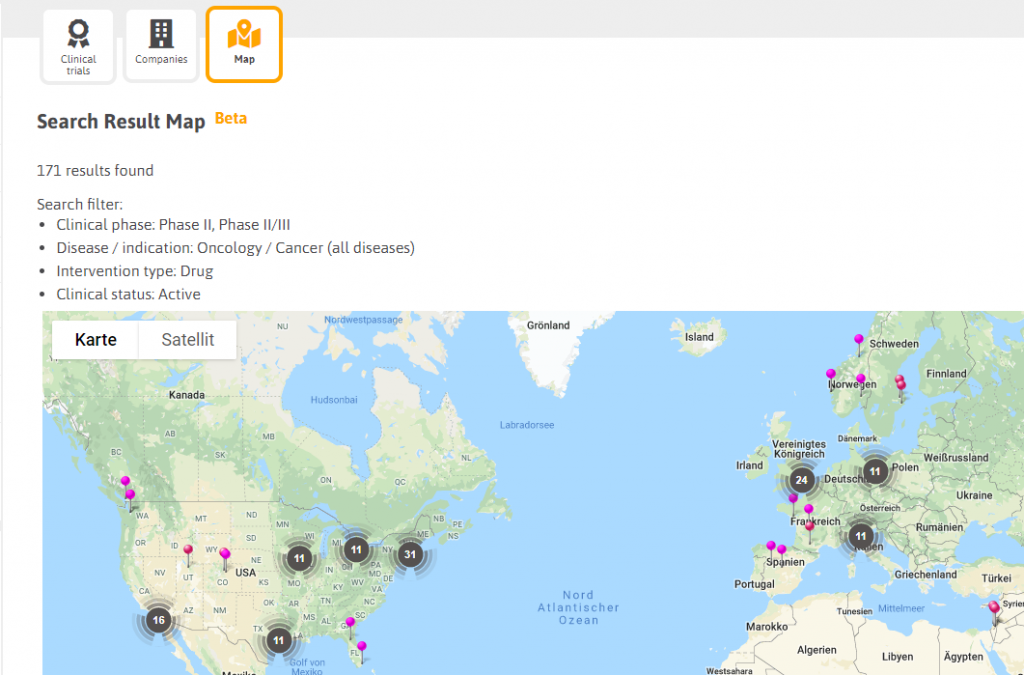
For any sections you search, and for any results you receive, you can change the view of the results so that you can see the list of related companies and a map with pins of their locations. As an example: Let’s assume you search for clinical trials of cancer drugs in phase II or phase II/III with clinical status “active”. Biotechgate will then show you the list of trials found. In addition to this, you can click on the “Companies” tab to see the related (sponsoring) company for the trial. The tab “Map” finally shows all companies on a map. This feature was requested by our clients and can be very useful when planning a road trip.
What other new features can we expect?
We implemented some enhancements to the “saved search & alerts” feature: It is now possible to save searches and setting up email alerts for any section. And all email alerts now include a link that allows downloading the reported details to excel. Also, this was requested by our clients. Besides we will move the entire application to a new platform which will have a dramatic impact on the performance. Thus, searches will be faster, the loading time will be reduced.
You mentioned in the beginning that all search forms have been re-developed. What exactly does this mean?
Overall we just made the search pages much more user-friendly. A key point in this matter is the introduction of the tagged search: Instead of selecting options from a long pull-down list you can just type in your keywords and the system will come up with suitable suggestions. As an example, if you search for licensing opportunities in North & Central America of specific lung cancer disease, you just start entering the keywords for the region and the indications into the text field and the system will come up with suggestions which you can select with an easy click. In addition, we improved the possibilities for different types of geographic filtering (combined search for regions, countries, and states) and added user-friendly sliders when searching for a value range, e.g. when searching for a specific range of milestone payments in the section license agreements.
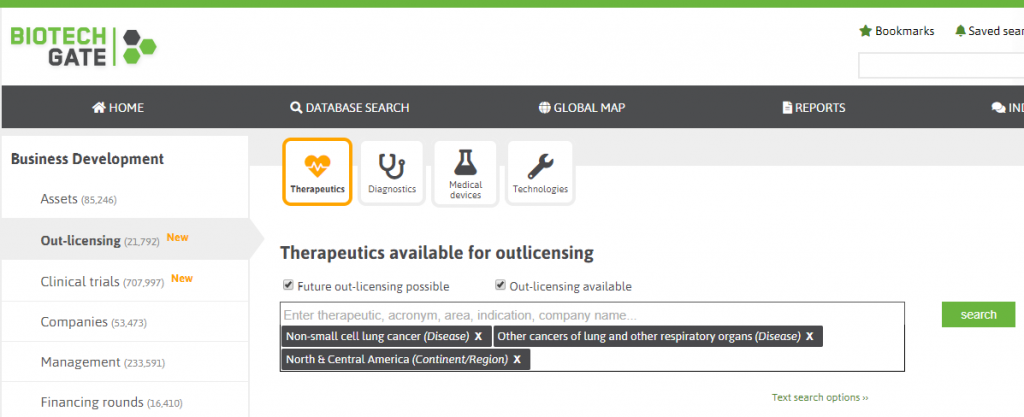
Screenshot 3: Tags for indications and region selected when searching for therapeutics available for out-licensing
What does the new release mean for existing subscribers? Do they need to learn a new application?
When developing the new Biotechgate version we made sure that we use the same “look and feel” from the previous version. Even though a lot has been changed and improved, the overall handling is still the same and as easy to use as the previous application. So there is no need for special training sessions. In case there is a question, our support staff is happy to help anytime.
Can I test the new Biotechgate features? And where can I learn more about it?
We are constantly writing articles and will provide short movies with tutorials about the different features. So it is worth to visit the Biotechgate resource center once in a while. However, the best way to see Biotechgate in action is to arrange a demo or a trial. Just use our contact form and our staff will be happy to get in touch with you: https://www.biotechgate.com/trial