In March 2019 we will launch the beta versions of two new components for Biotechgate: Clinical trials information and a new license agreement section. More than 450,000 clinical trials will be available in Biotechgate and more than 100’000 of them will be linked to existing company profiles in the database. In addition, a completely newly designed and very user-friendly license agreements section will be launched. The new components were made possible through an IT project that combines Big Data with artificial intelligence to acquire and extract relevant and timely information.
The goal of Biotechgate is to structure relevant data from the life science industry and make it easily accessible and searchable for its clients. With a new section for clinical trial data created from registries around the world and a completely revised licensing agreement section, Biotechgate will significantly enrich its offer. Data points such as clinical phase, status, and disease/indication areas are put into categories, providing highly accessible and relevant content for users. In addition, a new search framework has been implemented that suggests tags for different categories based on keywords entered by a user.
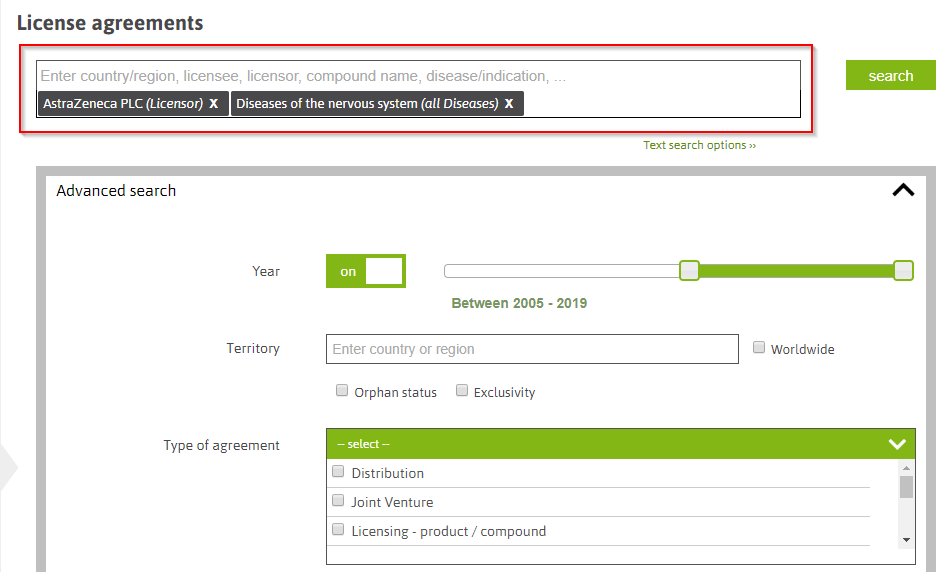 Search by tags: A user can enter keywords and select from suggested tags for licensor, licensee, compound names, phase etc.
Search by tags: A user can enter keywords and select from suggested tags for licensor, licensee, compound names, phase etc.
Big Data in combination with Artificial Intelligence
A significant challenge with working with large and adapting data is to find and extract the relevant information and make it available within a critical timeframe. To make this possible a project called DISCOVER was initiated in 2016 in collaboration with the Swiss Institute for Information Research (SII) in Chur, Switzerland. The project was funded by Innosuisse and Venture Valuation and has lasted for more than two years. DISCOVER combines “Big Data” with artificial intelligence generating efficient and automated acquisition, extraction and integration of decision-relevant information from heterogeneous online sources. This even allows for the retrieval of information that is often not covered by regular search engines. This data sources – also known as “Deep Web” – contain often very relevant and industry-specific information.
Thanks to DISCOVER the clinical trial registry will provide up-to-date information for users. In addition, new features will be implemented soon for other, existing, sections in Biotechgate: Our team of analysts will use DISCOVER for more efficient and faster updates and additions of financing rounds, changes in company management and M&A activities.
Interested?
Do you want to join our beta test for the new sections in Biotechgate? We are looking forward to offering you trial access to Biotechgate.


